Epatite C: aggiornamento del 21 luglio 2025 sui pazienti arruolati
L’Agenzia Italiana del Farmaco rende disponibile l’aggiornamento settimanale dei dati relativi ai trattamenti con i nuovi farmaci ad azione antivirale diretta di seconda generazione (DAAs) per la cura dell’epatite C cronica, raccolti dai Registri di monitoraggio AIFA.
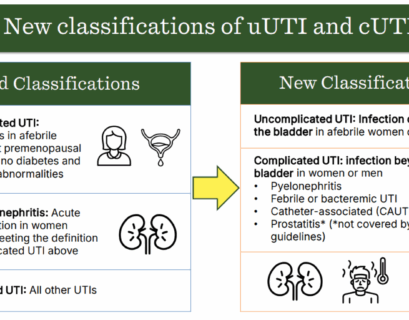
Linee guida sul trattamento e gestione delle infezioni complicate del tratto urinario (UTI)
UrologiaL’Infectious Diseases Society of America (IDSA) ha pubblicato le prime linee guida sulla gestione e il trattamento delle infezioni...
Linee guida per la sindrome genitourinaria della menopausa
Salute della DonnaUna nuova linea guida multi-organizzativa presenta 26 raccomandazioni per il riconoscimento e la gestione della sindrome genitourinaria...
Gestione delle infezioni sessualmente trasmissibili asintomatiche: linee guida
Malattie sessualmente trasmissibiliLe linee guida dell’OMS per la gestione delle infezioni sessualmente trasmissibili asintomatiche sottolineano l’importanza di...
Tumori della pelle: aumentano i casi tra i giovani in Italia
Melanoma – Melanoma e tumori della pelle in aumento, ma nuove terapie riducono recidive e migliorano la sopravvivenza. Controlli dermatologici ancora troppo scarsi.
Linea guida sulla gestione della malattia venosa cronica
CardiovascolareLa Society for Cardiovascular Angiography and Interventions (SCAI) ha pubblicato una linea guida sulla gestione della malattia venosa...
Scoperta cura efficace…ma chissà per quando
Comunicazione – L’annuncio della scoperta di cure efficaci può creare false speranze nei pazienti che soffrono e nei loro parenti: informazione utile o titoli acchiappa clic?
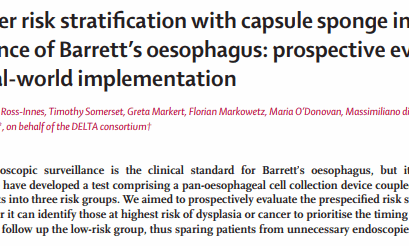
La “pillola su filo” per monitorare l’esofago di Barrett riduce le endoscopie
Notizie dalla RicercaUn nuovo test con capsula e spugna, molto meno invasivo, per rilevare i primi segni di cancro potrebbe sostituire in modo sicuro l’uso...
Cos’è l’acido ialuronico e a cosa serve?
Acido ialuronico – Tutto quello che devi sapere sul filler con acido ialuronico: in quali zone si può fare, durata, effetti collaterali e raccomandazioni del medico estetico.
L’agopuntura facilita il recupero postoperatorio dopo gastrectomia
Stomaco – L’agopuntura si è dimostrata efficace nel recupero dei pazienti con gastrectomia: uno studio sul post-operatorio nel tumore allo stomaco.
Linee guida sul parto cesareo
Salute della DonnaQueste linee guida aggiornate riguardano il parto cesareo, gli aspetti procedurali dell’intervento e l’assistenza post-cesareo. Mirano...
Gestione dell’obesità negli adulti con insufficienza cardiaca: linea guida
CardiovascolareLa dichiarazione scientifica del 2025 dell’American College of Cardiology (ACC) approfondisce la gestione dell’obesità negli adulti...
Gestione del morbo di Crohn negli adulti: linee guida
Gastroenterologia ed EpatologiaL’articolo fornisce linee guida complete per la diagnosi, gestione e trattamento del morbo di Crohn (CD) negli adulti. Include...
Alzheimer: test del sangue svolta epocale per diagnosi tempestive
Notizie dalla RicercaLa FDA approva un test ematico per la diagnosi precoce dell’Alzheimer, che individua biomarcatori come beta-amiloide e tau fosforilata....